Public domain

Living organisms are constantly subjected to agressions, such as molecular attacks, damaging cells or biological macromolecules.
When molecules (or atoms) are missing electrons, they become unstable and very reactive. ROS are molecules derived from the oxidation-reduction process. During reduction, a substance gains electrons from a reducing agent, named an "anti-oxidant". A molecule that loses its electron (and thus gains in oxygen) is called a "pro-oxidant,". In response, those reactive oxygen species reacts to their environment and binds to proteins which causes changes in their structure and/or function. They also react with cell membranes, causing damage. In some cases, DNA may also be affected, causing mutations to the genome. Excessive oxidative stress damage can result of the cell.
There are several ROS, but what these molecules have in common is that they contain oxygen and are unstable, thus highly reactive. Examples of free radicals (or leading to) are:
hydroxyl (OH-),
superoxide (O2-)
nitric oxide (NO)
peroxynitrite (ONOO),
hydrogen peroxide (H2O2)
lipid peroxides (LOOH).
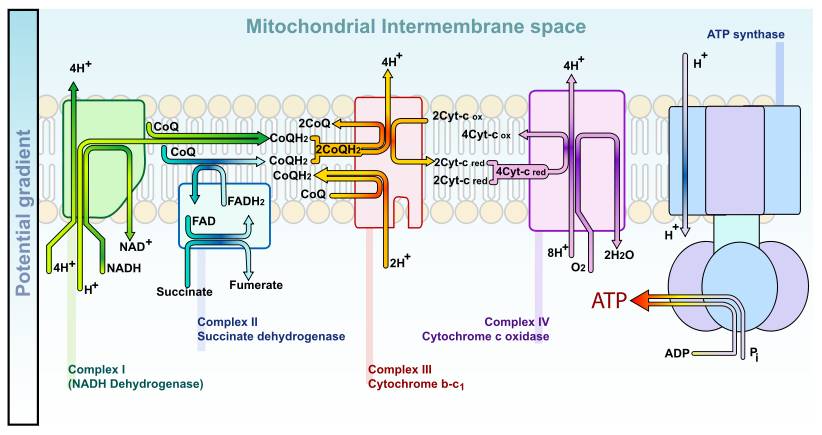
There are several types of ROS, but all these molecules contain oxygen and are highly reactive. They can interact with each other, creating more of these damaging molecules. ROS are naturally produced by endogenous mechanisms in a living organism. Cellular respiration, the energy generator of the cell, requires oxygen to function. During this process, oxygen is transformed into ROS. Nitric oxide is produced by nitric oxide synthase (NOS) and is implicated in blood vessel’s vasodilatation. Moreover, the immune system uses ROS to defend itself against microbes. Phagocytic cells (macrophages, microglia) use ROS as a first defense line in innate immunity (“oxidative burst”). ROS are produced by NADPH oxidase family members, and are involved in apoptosis activation, cytokines production, inflammatory and anti-microbial reaction. Finally, ROS may come from other sources, such as food or other elements (smoking, pollution, ...)
Many enzymes involved in the generating of ROS like:
NADH dehydrogenase complex
complex III (cytochrome bc1)
monoamine oxidases
succinate dehydrogenase
aconitase
a-ketoglutarate dehydrogenase.
Nitric oxide is produced by nitric oxide synthase (NOS). In addition, phagocytic cells use ROS as a first defense line in innate immunity ("oxidative burst"). ROS are produced by NADPH oxidase family members, and are involved in apoptosis activation, cytokines production, inflammatory and anti-microbial reaction. Finally, ROS may come from other sources, such as food or other elements (smoking, pollution, ...)

Public domain
ROS are highly reactive, and can create chemical changes in macromolecules. Cell membranes are prone to peroxidation. DNA and RNA are subject to mutations. Many protein modifications such as nitration or carbonylation can damage proteins causing dysfunction or damage. Some aldehydic products (4-hydroxy-2-nonenal, HNE) get attached to proteins. Too many modifications can cause cell apoptosis.
The body defends itself against these ROS, because large quantities are harmful. To protect the cells, ROS are eliminated by transformation into a simple water molecule, for instance through superoxide dismutase and catalase. The glutathione system, an antioxidant molecule trapping ROS, is resplenished by the action of glutathione reductase and peroxidase. Also, various redoxines reduce peroxide molecules, thus eliminating their oxidant power. Vitamins C and E, polyphenols (red wine), carotenes (fruits and vegetables), flavones (soybeans) and omega3 fatty acids (fish, nuts) are other anti-oxidant molecules.
Many antioxidant molecules are found in mitochondria:
vitamin E and coenzyme Q10: trapping ROS
cytochrome C molecules: removing produced superoxide
glutathione (GSH): maintaining balance of intracellular redox thiol groups.
Oxidative stress is defined as a imbalance between antioxidant and oxidant molecules. This may arise due to an increased production or a decreased removal of ROS. For example, an infection or illness may lead to excessive oxygen consumption and result in an overproduction of ROS. Furthermore, declining or dysfunctional antioxidant molecules may also result in an increase of ROS.
It is believed that oxidative stress plays an important role in the pathogenesis of hepatic encephalopathy. A review of Dr Rose was written on this subject (Bosoi and Rose, 2013). We have shown that ammonia in association with oxidative stress lead to cerebral edema in rats with chronic hepatic failure (Bosoi et al, 2012 and Bosoi et al, 2014). However, hyperammonemia alone is not sufficient to induce edema (Yang et al, 2010)
Moreover, ammonia, by reacting with oxygen, create nitric oxide and peroxynitrite, reacting easily with tyrosine. Those nitrotyrosine was observed on glutamine synthetase, the enzyme responsible of ammonia detoxification in brain, then reducing its efficiency (Schliess et al, 2002). Nitrotyrosine were also detected in cirrhotics patients with minimal hepatic encepalopathy (Montoliu et al, 2011).